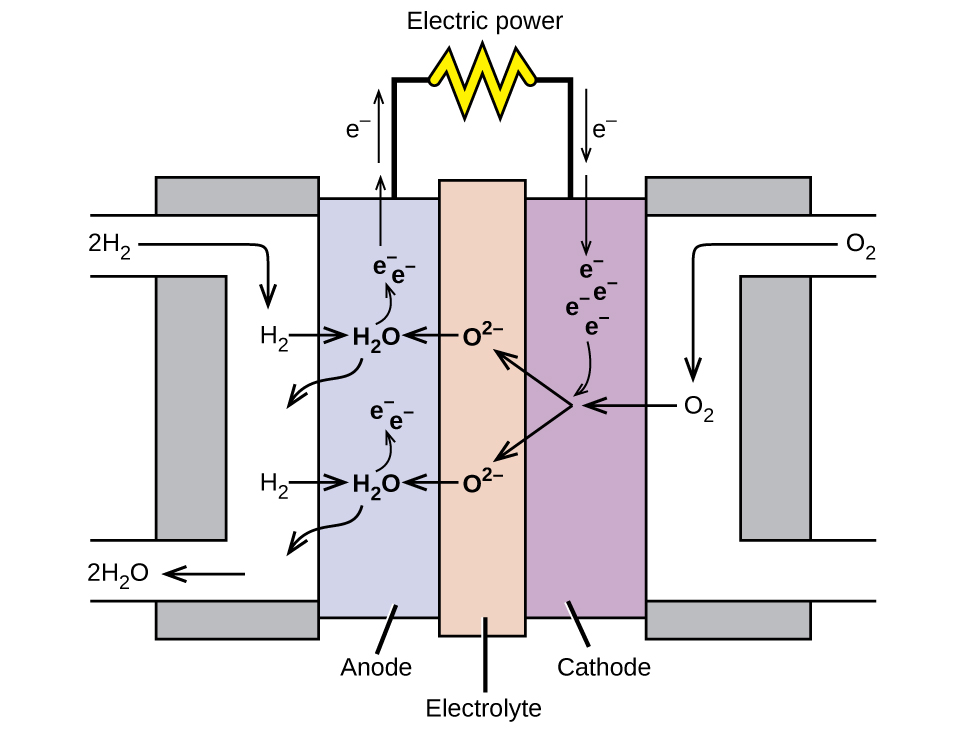
Definition Of Hydrogen Fuel Cells In Chemistry . In many ways fuel cells are similar to batteries, such as those. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Oxygen is supplied to a similar. A fuel, such as hydrogen, is. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen.
from chem.libretexts.org
A fuel, such as hydrogen, is. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. In many ways fuel cells are similar to batteries, such as those. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity.
6.7 Batteries and Fuel Cells Chemistry LibreTexts
Definition Of Hydrogen Fuel Cells In Chemistry a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Oxygen is supplied to a similar. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. A fuel, such as hydrogen, is. In many ways fuel cells are similar to batteries, such as those.
From www.chfca.ca
About Fuel Cells CHFCA Definition Of Hydrogen Fuel Cells In Chemistry A fuel, such as hydrogen, is. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. In many ways fuel cells are similar to batteries, such as those. Oxygen is supplied to a similar. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. . Definition Of Hydrogen Fuel Cells In Chemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Definition Of Hydrogen Fuel Cells In Chemistry hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In many ways fuel cells are similar to batteries, such as those. a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Definition Of Hydrogen Fuel Cells In Chemistry A fuel, such as hydrogen, is. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Oxygen is supplied to a similar. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In many ways fuel cells. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记6.1.8 Fuel Cells翰林国际教育 Definition Of Hydrogen Fuel Cells In Chemistry a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. Oxygen is supplied to a similar.. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.simscale.com
Hydrogen Fuel Cell Simulation & Modeling Blog SimScale Definition Of Hydrogen Fuel Cells In Chemistry a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell consists. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Definition Of Hydrogen Fuel Cells In Chemistry A fuel, such as hydrogen, is. Oxygen is supplied to a similar. hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In many ways fuel cells are similar to batteries, such as those. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.youtube.com
Hydrogen & Fuel Cells Reactions Chemistry FuseSchool YouTube Definition Of Hydrogen Fuel Cells In Chemistry Oxygen is supplied to a similar. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuel cell, any of a class of devices that convert the chemical energy. Definition Of Hydrogen Fuel Cells In Chemistry.
From guidefixmuiceta.z22.web.core.windows.net
Working Of Hydrogen Fuel Cell With Diagram Definition Of Hydrogen Fuel Cells In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. In many ways fuel cells are similar to. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.w3schools.blog
Hydrogen as a fuel W3schools Definition Of Hydrogen Fuel Cells In Chemistry In many ways fuel cells are similar to batteries, such as those. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel, such as hydrogen, is. a hydrogen fuel cell converts chemical energy stored. Definition Of Hydrogen Fuel Cells In Chemistry.
From mungfali.com
Hydrogen Fuel Cell Chart Definition Of Hydrogen Fuel Cells In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. In many ways fuel cells are similar to batteries, such as those. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Oxygen is supplied to a. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Definition Of Hydrogen Fuel Cells In Chemistry A fuel, such as hydrogen, is. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. Oxygen is supplied to a similar. In many ways fuel cells are similar to batteries, such as those. a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. fuel cell, any of a class of. Definition Of Hydrogen Fuel Cells In Chemistry.
From specialgasinstruments.com
Hydrogen Fuel Cell Definition, Types, & Use Cases Special Gas Definition Of Hydrogen Fuel Cells In Chemistry hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Oxygen is supplied to a similar. hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. a fuel cell consists. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.gecos.polimi.it
Hydrogen, Fuel Cells and Electrochemical Energy Systems GECOS page Definition Of Hydrogen Fuel Cells In Chemistry a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. A fuel, such as hydrogen, is. In many ways fuel cells are similar to batteries, such as those. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative. Definition Of Hydrogen Fuel Cells In Chemistry.
From exozpzccv.blob.core.windows.net
Fuel Cell For Chemical Reaction at Lydia Hatcher blog Definition Of Hydrogen Fuel Cells In Chemistry a hydrogen fuel cell converts chemical energy stored by hydrogen fuel into electricity. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel, such as hydrogen,. Definition Of Hydrogen Fuel Cells In Chemistry.
From thechemistrynotes.com
Fuel Cell Definition, Types, and Advantages Definition Of Hydrogen Fuel Cells In Chemistry a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Oxygen is supplied to a similar. A fuel, such as hydrogen, is. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a hydrogen fuel cell converts chemical. Definition Of Hydrogen Fuel Cells In Chemistry.
From mungfali.com
Fuel Cell Schematic Definition Of Hydrogen Fuel Cells In Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. Oxygen is supplied to a similar. In many ways fuel cells are similar to batteries, such as those. a fuel cell consists of. Definition Of Hydrogen Fuel Cells In Chemistry.
From www.eseslab.com
Hydrogen Storage Definition Of Hydrogen Fuel Cells In Chemistry Oxygen is supplied to a similar. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel, such as hydrogen, is. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. fuel cell, any of a class of devices that convert the chemical energy of. Definition Of Hydrogen Fuel Cells In Chemistry.
From wiringlibrichter.z19.web.core.windows.net
Hydrogen Fuel Cell Diagram Car Definition Of Hydrogen Fuel Cells In Chemistry a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In many ways fuel cells are similar to batteries, such as those. A fuel, such as hydrogen, is. hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. fuel cell, any of a class. Definition Of Hydrogen Fuel Cells In Chemistry.